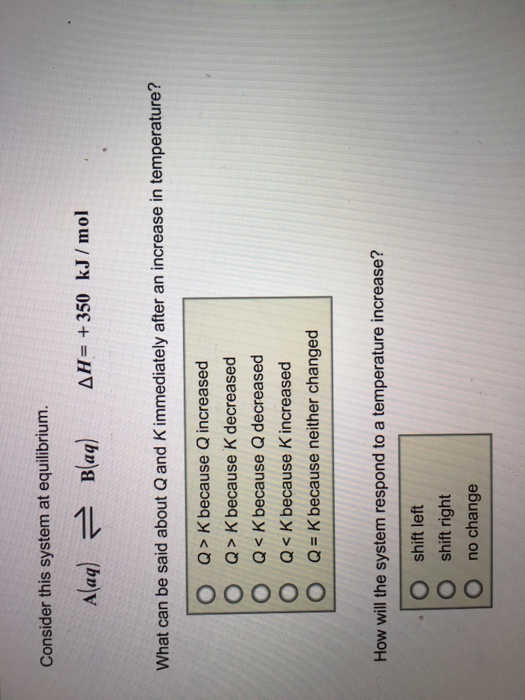
Consider This System At Equilibrium A Aq B Aq
Consider this system at equilibrium a aq b aq. Consider this system at equilibrium. Part A Equilibrium and an Acid Base Indicator Equilibrium system HA aq H aq A aq from CHEM 12 at Santa Monica College. Q K because K decreased.
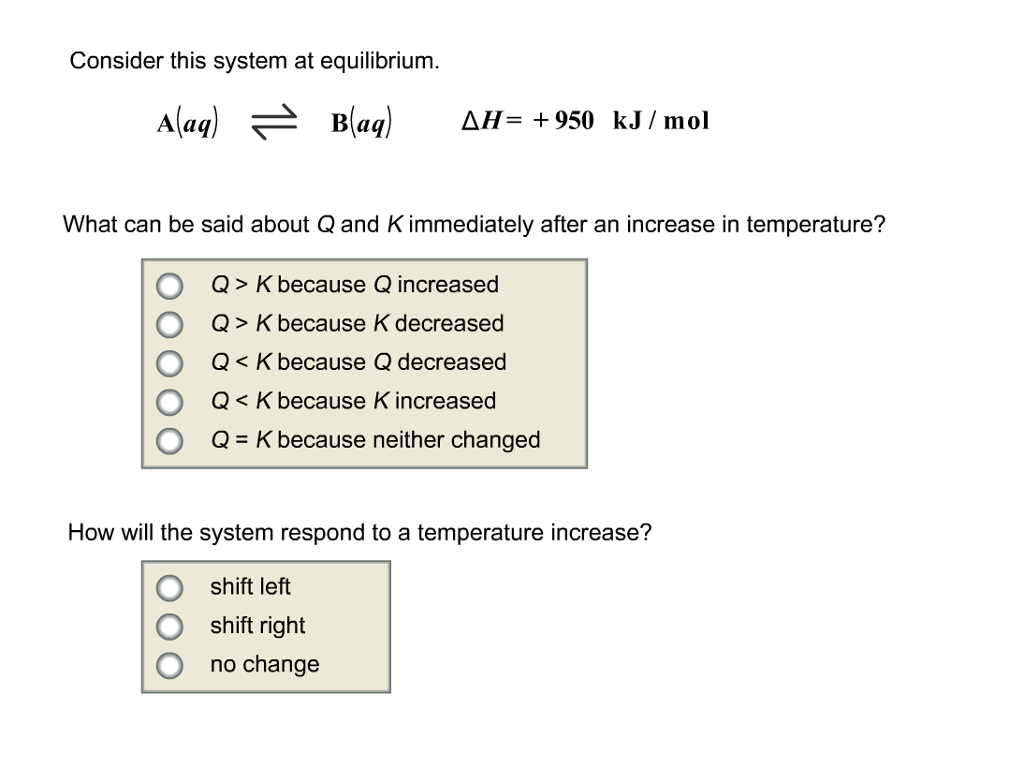
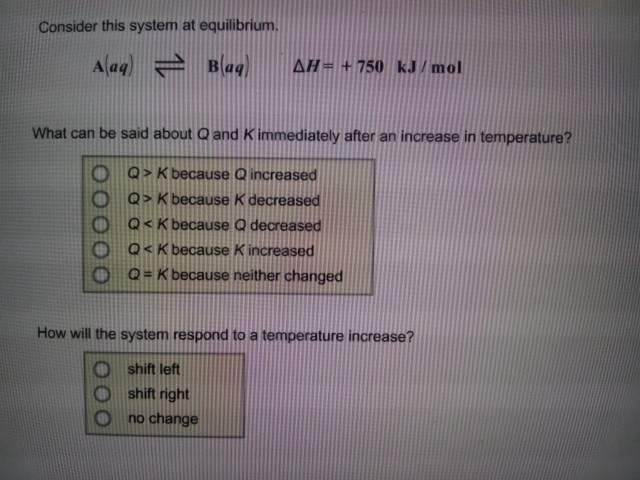
Q K bc neither changed. A a q B a q d e l t a H 750 k J m o l. Consider this system at equilibrium.
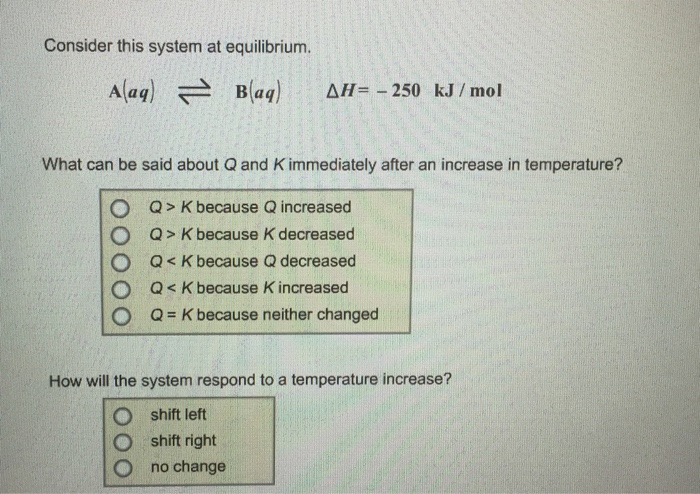
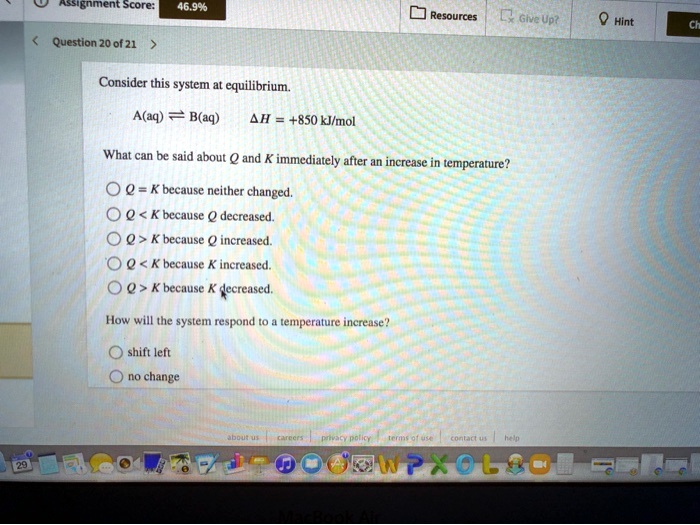
C Q. Consider this system at equilibrium. A aq B aq Δ H 850 kJmol What can be said about Q and K immediately after an increase in temperature.
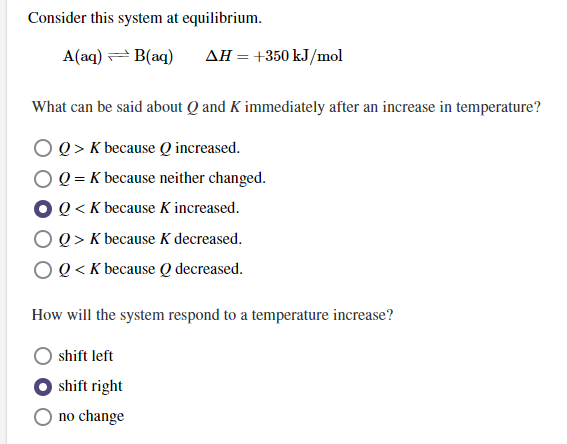
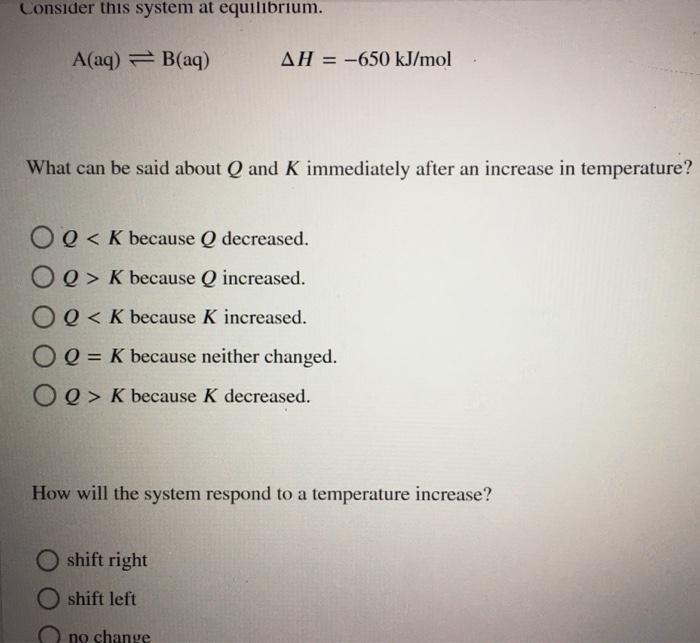
285 Bought 3 Share With. Aaq Baq ΔH 450 kJmol a What can be said about Q and K immediately after an increase in temperature. A aqlt--- gt B aq deltaH-750 kJmol Aaq BaqdeltaH 750kJ mol.
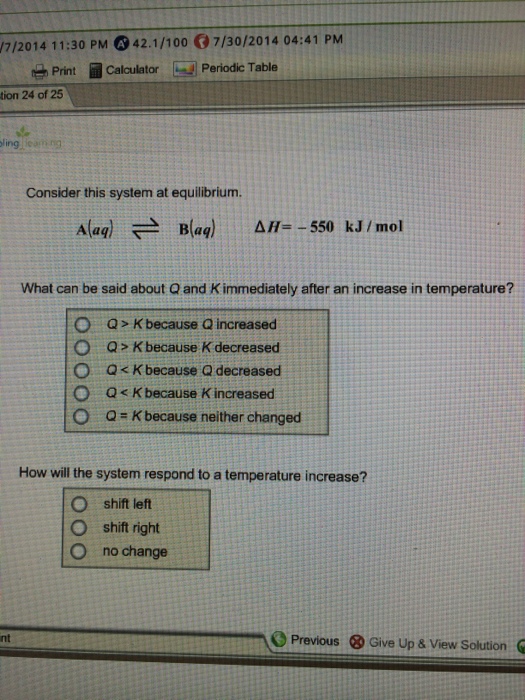
Q K bc K increased. 2 on a question Consider this system at equilibrium. What can be said about Q and K immediately after an increase in temperature.
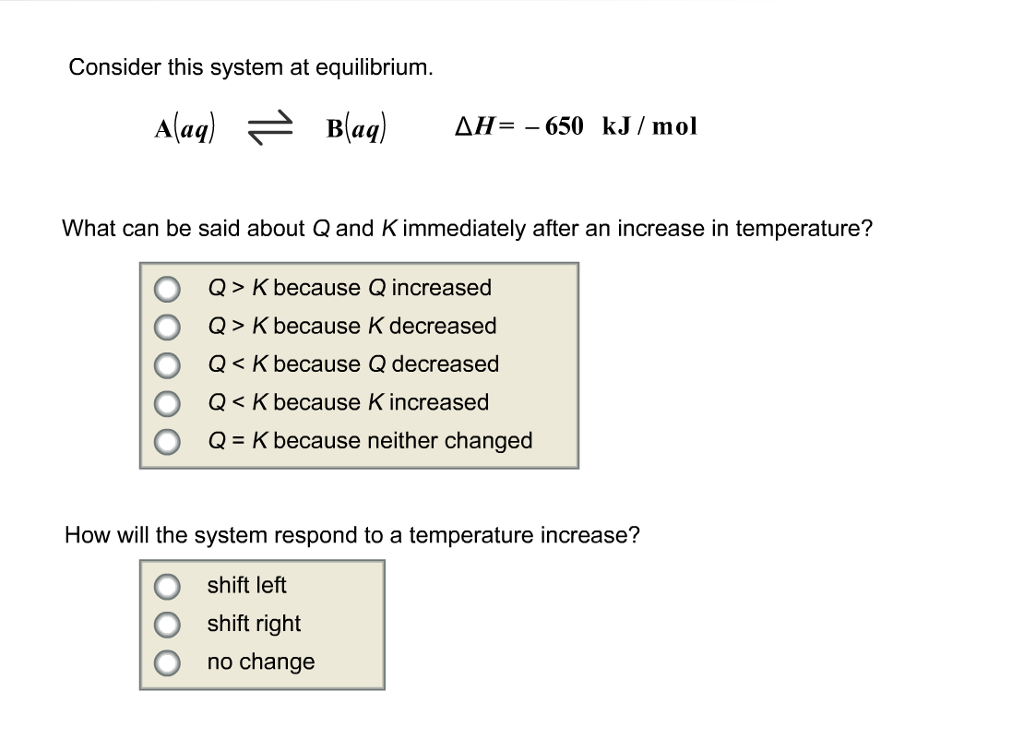
Consider this system at equilibrium. Q K bc Q decreased. Aaq Baq Delta H 750 kJmol.
AaqBaq deltaH-750 kJmol What can be said about Q and K immediately after an increase in temperature. Start studying CHEM 101 L 24-29.
Consider this system at equilibrium.
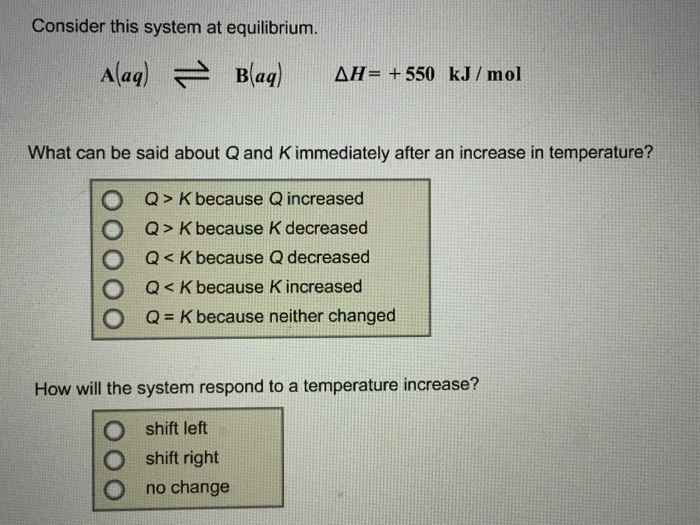
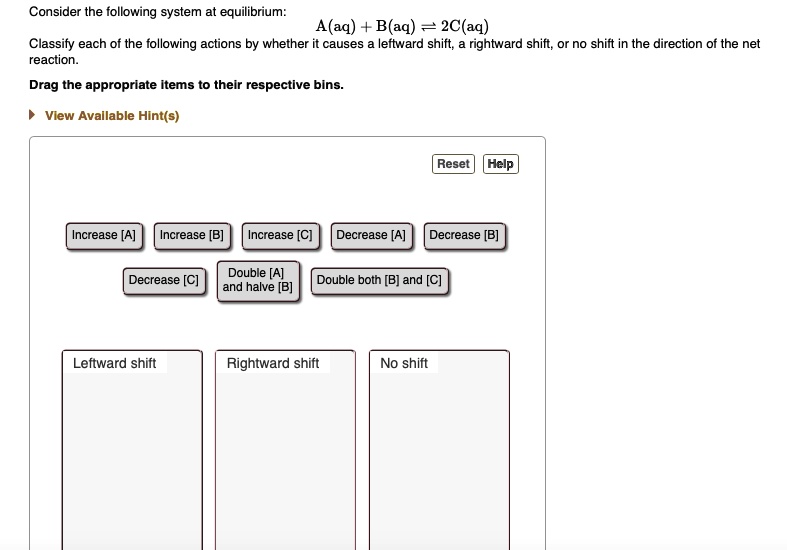
Start studying CHEM 101 L 24-29. A a q B a q d e l t a H 750 k J m o l. Consider the following system at equilibriumCaCl2 s Ca2 aq 2Cl- aq1 How will adding more CaCl2 shift the equilibrium. 2 question Consider this system at equilibrium. A to the right b to the left c no effect 2 How will removing some Ca2 shift the equilibrium. Consider this system at equilibrium. Consider this system at equilibriumA aq B aq ΔH -750 kJmolWhat can be said about Q and K immediately after an increase in temperatureA Q K because Q increasedB Q K because K decreasedC Q K because Q decreasedD Q K because K increasedE Q K because neither changed How will the system respond to a temperature. A aqB aq Δ𝐻850 kJmol What can be said about 𝑄 and 𝐾 immediately after an increase in temperature. Consider this system at equilibrium.
Consider this system at equilibrium. A Q K because Q increased. A aqB aqΔH750 kJmol A aqB aqΔH750 kJmol What can be said about Q and K immediately after an increase in temperature. Consider this system at equilibrium. A aqlt--- gt B aq deltaH-750 kJmol Aaq BaqdeltaH 750kJ mol. 2 question Consider this system at equilibrium. Aaq Baq Delta H 750 kJmol.




















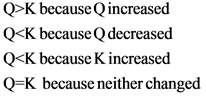


Post a Comment for "Consider This System At Equilibrium A Aq B Aq"